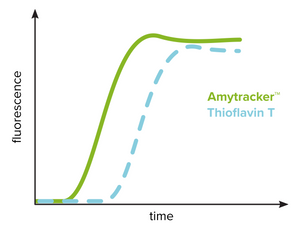
The kinetics of amyloid formation from conformational conversion of a peptide or protein into its fibrillar form (amyloid) is studied using fibrillation assays using a spectrophotometer. This technique requires extremely low background fluorescence of the unbound probe and Thioflavin T has been widely used for this reason. The kinetic profile typically includes a lag phase that is followed by a rapid exponential growth phase and a plateau phase. As shown in the figure, Thioflavin T can reliably identify the presence of amyloid fibrils but are limited in detecting prefibrillar aggregates. Amytracker binds and detects prefibrillar aggregates present during the initial lag phase as well as mature amyloid fibrils. Therefore, the lag phase is shortened significantly when investigating amyloid formation using Amytracker. Amytracker has been used to study fibrillation of recombinant Aβ1-40 and Aβ1-42 as well as lysozyme and insulin.
Read More:
- Åslund, A. et al. (2009) Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol, 4, 673-684
- Hammarström, P. et al. (2010) A fluorescent pentameric thiophene derivative detects in vitro-formed prefibrillar protein aggregates. Biochemistry, 49, 6838-6845
- Klingstedt, T. et al. (2012) Synthesis of a library of oligothiophenes and their utilization as fluorescent ligands for spectral assignment of protein aggregates. Org Biomol Chem, 9, 8356-8370
- Klingstedt, T. et al. (2013) The structural basis for optimal performance of oligothiophene-based fluorescent amyloid ligands: conformational flexibility is essential for spectral assignment of a diversity of protein aggregates Chemistry, 19(31), 10179–10192
