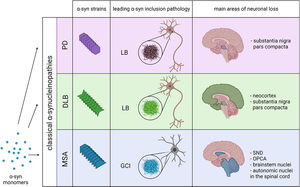
Alpha-synuclein (α-Syn) is a presynaptic neuronal protein encoded by the SNCA gene. It is expressed heavily in the brain and regulates synaptic vesicle trafficking and subsequent neurotransmitter release. Under certain conditions, α-Syn aggregates and forms, together with other components, intracellular inclusions called Lewy bodies (LBs) or Lewy Neurites (LN), which are the defining pathological hallmark of many synucleinopathies. Several familial mutations of the SNCA gene have been identified causing LB and LN pathology, but manifest in different diseases like Parkinson's Disease, Dementia with Lewy bodies (DLB) or Multiple System Atrophy (MSA), suggesting that the various mutations may act via distinct mechanisms.
Image: Various pathologies like Parkinson’s Disease (PD), Dementia with Lewy Bodies (DLB) and Multiple Systems Atrophy (MSA) are caused by different α-Syn strains. Image from Figure 1 by Malfertheiner, K. et al. (2021) Frontiers in Neurology, 12, 737195 (CC BY).
In a study published in Science Advances, Senthilt Kumar and colleagues from Hilal A. Lashuel’s group at the EPFL, Switzerland, provided an in-depth characterization of a novel SNCA mutation which is unique as it is the first one that is located in the Non-Amyloid Component (NAC) domain. The patient carrying this mutation presented with DLB and atypical frontotemporal lobar degeneration. The researchers produced recombinant wild-type (wt) and mutant E83Q α-Syn and showed that the E83Q mutation accelerates the aggregation kinetics of α-Syn and that fibrils of α-Syn show distinct morphology, stability, and structural features in vitro. Since α-Syn is a membrane protein the researchers wondered if the E83Q mutation influences membrane interaction. Using artificial vesicle and cellular models they show that the E83Q mutation does not strongly alter α-Syn binding to lipid vesicles or intracellular membranes and does not promote the formation of pathological α-Syn aggregates in mammalian cell lines. However, a greater propensity of E83Q α-Syn to form cytotoxic oligomers and higher seeding activity was established. Interestingly, the researchers were able to show that seeding with E83Q α-Syn preformed fibrils, but not WT α-Syn preformed fibrils leads to formation of Lewy Body-like inclusions in neurons. While classical markers were used to characterise the protein components, Amytracker was valuable to determine the amyloid character within these inclusions. Taken together, the study by Kumar et al. provides an impressively complete picture on how a single mutation in the SNCA gene can unlock the pathogenicity of human α-Syn fibrils and showcases how the pathological diversity of synucleinopathies might be related to specific strains of α-Syn fibrils.
