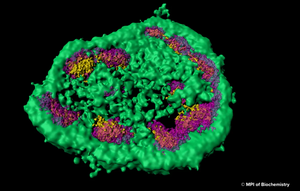
The nuclear proteome is rich in proteins which are prone to aggregate upon conformational stress. This might explain why intranuclear inclusions can often be found in neurodegenerative disorders associated with protein aggregation. Using a combination of fluorescence imaging, biochemical analyses, and proteomics, researchers at Max Planck Institute for Biochemistry around Prof. F.-Ulrich Hartl have investigated the role of the nucleolus as a “phase-separated protein quality control compartment” and published their results in the renowned scientific journal Science. The nucleolus is the largest non–membrane-bound nuclear subcompartment and consists of liquid-like phases that do not intermix, giving rise to distinct zones. To investigate the fate of a nuclear protein during heat stress, the researchers generated a cell line producing a reporter protein called NLS-LG. NLS-LG carries a nuclear localization signal, to make sure it is distributed to the nucleus. The protein's location can be tracked by a heat stable fluorescent signal and its folding state can be tracked with a thermolabile fluorescent signal. When the researchers exposed the cells to heat, they saw that NLS-LG was unfolded and transferred into the outermost zone of the nucleolus. (Figure: The outermost zone of the nucleolus is the granular component (GC) phase marked in green.) Upon recovery from heat stress, NLS-LG was again relocated to the nucleus and a bright fluorescent signal indicated successful refolding. It was further shown that Hsp70, which is an important part of the cell's machinery for protein folding, also transferred to the nucleolus upon heat stress. When the activity of Hsp70 was inhibited, relocation of NLS-LG to the nucleus after recovery from heat stress was also prevented.
Image: Super-resolution light microscopy shows that the nucleolus is not separated from the rest of the cell by a membrane and that it consists of different zones, which are distinct from each other and membrane-less as well. © MPI of Biochemistry)
When nucleolar organization was disrupted by a toxin that causes nucleolar disassembly and the cells were exposed to heat, instead of translocating to the nucleolus, the NLS-LG reporter protein formed aggregate foci in the nucleus. Using Amytracker 680, the authors were able to demonstrate that these nucleoplasmic foci, in contrast to nucleolar assemblies, were positive for amyloids with a cross β structure. Although recovery from heat stress was slow and inefficient in the nucleoplasmic foci, a certain capability of refolding was evident since Amytracker 680 fluorescence intensity decreased upon recovery from heat stress. This demonstrates that Amytracker 680 can also be used to label reversible aggregates on top of irreversible amyloid fibrils. To explore the protective capacity of nucleolar quality control, cells were exposed to prolonged heat stress. This led to an increase in nucleolar volume attributed to the influx of misfolded protein and to a transition in nucleolar appearance from liquid droplet-like to a hardened state. When Amytracker 680 was used under these conditions in cells expressing the NLS-LG reporter protein, a distinct nucleolar signal indicated presence of amyloids with a cross β structure, which dissolved slowly upon recovery from heat stress. This shows the potential of Amytracker 680 to report the presence of amyloids in phase separated compartments like nucleoli highlighting Amytracker as a tool to be used in in vitro liquid-liquid phase separation experiments for studying protein aggregation and amyloid formation.
Taken together, the publication by Frottin et al. pioneers the notion that “the nucleolus serves as a storage compartment for a subset of misfolded proteins under proteotoxic stress conditions, preserving them in a state competent for refolding or degradation”. The impairment of nucleolar quality control through environmental stressors and other factors might directly contribute to the emergence of idiopathic neurodegenerative pathology.
