Neurodegenerative diseases often involve the accumulation of misfolded proteins, that cells fail to refold or degrade and that eventually form aggregates. Among these, oligomers, which are small, still-soluble aggregates, are considered the most toxic and can lead to neuronal death. Identifying the aggregation-prone regions of these oligomer forming proteins is key for developing new therapeutic approaches.
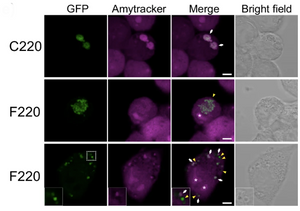
In over 97% of amyotrophic lateral sclerosis (ALS) cases, TDP-43 (TAR DNA/RNA-binding protein 43) condensates accumulate in the cytoplasm. The carboxy-terminal region of TDP-43 is processed into smaller aggregation-prone fragments that are included into these condensates. Akira Kitamura and their colleagues at Hokkaido University explored these fragments by expressing TDP-43 deletion constructs in murine neuroblastoma cells (N2A) and C. elegans. They found that TDP25, a fragment containing a glycine-rich domain and part of the RRM2 RNA-binding domain, is necessary and sufficient for condensate formation. The glycine-rich region drives this process, while the remaining N-terminal fragment is highly aggregation-prone. Only some of these condensates were stained by Amytracker 680, and therefore had an amyloid-like structure. This suggests that multivalent interactions and conformational changes in biomolecular condensates might facilitate the formation of amyloid aggregates of TDP-43. Expression of TDP25 caused cell death in N2A cells and reduced lifespan in C. elegans, suggesting this fragment contributes to the neurotoxicity observed in ALS. Therefore, stabilising TDP25 could be a potential therapeutic target to prevent toxic TDP-43 oligomers.
Image: Confocal images of N2A cells expressing different GFP-tagged TDP-43 carboxy-terminal fragments (green) stained with Amytracker 680 (magenta). In cells expressing the F220 fragment, not all the condensates formed are positive for Amytracker staining, suggesting that only a fraction of them contains amyloid fibrils. White arrows and yellow arrowheads represent the positions of Amytracker-positive and negative condensates in the cytoplasm. The asterisk in the images represents the nucleolus. Scale bar = 5 μm. Image from Figure 3E by Kitamura et al. (2024) Communications Biology Chemistry, 7(1), 743 (CC BY 4.0)
