Tau is an intracellular protein which associates with microtubules and stabilizes them. Physiologically, Tau is phosphorylated to facilitate release from the microtubules and thereby favor microtubule shortening. In a pathological state, Tau is hyperphosphorylated which increases its tendency to aggregate in the cytoplasm. This means that conditions which promote abnormal phosphorylation of Tau promote its aggregation. Aggregates of hyperphosphorylated Tau form insoluble filaments and tangled clumps. These intracellular deposits are called Neurofibrillary tangles (NFTs).
In the literature, predecessors of Amytracker have been used to label NFTs in tissue sections. It was found that the signal co-localises with the signal from monoclonal antibodies for phosphorylated Tau (AT8), thus confirming its specificity.
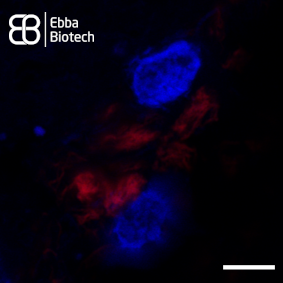
In cortical tissue sections showing pronounced AD pathology Amytrackers labels bona-fide intracellular aggregates and reveals a clear fibrillar morphology assembled in tangled net-like structures (see image).
Read More:
- Åslund, A. et al. (2009) Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol 4(8), 673-684
- Herrmann, U. et al. (2015) Structure-based drug design identifies polythiophenes as antiprion compounds. Sci Transl Med 7(299), 299ra123–299ra123
- Mahul-Mellier, A. et al. (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci USA 117(9), 4971-4982
- Moloney, C.M. et al. (2021) Visualization of neurofibrillary tangle maturity in Alzheimer's disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement 17, 1554-1574
