Aggregates of α-synuclein are the major component of Lewy bodies which are the pathological hallmark of a series of neurodegenerative disorders, called Lewy body diseases or Synucleinopathies. In physiological conditions, α-synuclein regulates synaptic vesicle-release and possibly cytoskeletal assembly. However, this small pre-synaptic protein is characterized by an intrinsically disordered structure that makes it prone to aggregation. When aggregated, α-synuclein can interact with membranes and, for instance, make neurotransmitter vesicles “leaky”. This is especially important in dopaminergic neurons, as the interaction between dopamine and α-synuclein seems to further facilitate its oligomerization. When aggregated, α-synuclein cannot be disposed of by the cellular recycling pathway, it is instead deposited into intracellular inclusion bodies together with other proteins.
An Amytracker-like molecule has been used to label α-synuclein aggregates amplified in cerebrospinal fluid from patients with Synucleinopathies such as Multiple Systems Atrophy and Parkinson’s Disease (Shahnawaz, M. et al, 2020). Further, Amytracker 680 has been used to confirm the amyloid nature of α-synuclein aggregates in a cellular fibril-seeding assay (Mahul-Mellier, A. et al. 2020).
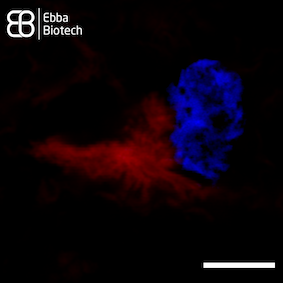
Superresolution images of thalamic tissue sections with pronounced PD pathology labeled with Amytracker 680 and DAPI counterstain show intracellular aggregates (red) located close to the nucleus (blue) in great detail (see image, scale bar 5 µm).
- Mahul-Mellier, A. et al. (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci USA 117(9), 4971-4982
- Frey, B. et al. (2021) Monitoring alpha-synuclein oligomerization and aggregation using bimolecular fluorescence complementation assays: What you see is not always what you get. J Neurochem 157(4), 872–888
- Shahnawaz, M. et al. (2020)Discriminating α-synuclein strains in Parkinson's disease and multiple system atrophy. Nature 578(7794), 273–277
