By 2050, the number of people aged >65 is expected to double. This will lead to a significant rise in the prevalence of age-related diseases. Many of these conditions are linked to two major processes: protein aggregation and chronic inflammation.
The precise relationship between these two components and how they contribute to the pathology of age-related diseases remains unclear. It is uncertain whether protein aggregation triggers inflammation, or if inflammation drives the aggregation process.
To date, anti-amyloid therapies, which were designed to reduce protein aggregation, have not delivered the hoped-for results in clinical trials. Instead, targeting the pro-inflammatory signalling pathways has shown promise at the pre-clinical level. It seems that this strategy could hold the key to developing effective therapies against neurodegenerative diseases.
One major challenge for researchers is the lack of tools to observe and visualise early stages of protein aggregation within live cells and organisms. A recent publication by Eltom et al. found that astrocytes can take up amyloid fibrils from their surroundings, but are unable to degrade them. Instead, the fibrils accumulate in the astrocytes' cytoplasm, causing the release of pro-inflammatory cytokines. Similar findings were reported by the Klenerman group at Cambridge University and the Bruns group at the University Hospital in Erlangen. They showed that macrophages activate pro-inflammatory signaling when exposed to either amyloid-β fibrils or β2-microglobulin (which accumulates in multiple myeloma). >Research from the Audas lab at Simon Fraser University demonstrated that anti-inflammatory drugs can reduce amyloid-β accumulation, further strengthening the relationship between inflammation and protein aggregation.
These findings underscore the value of Amytracker as a tool designed for live-cell studies which could provide crucial insights into the relationship between inflammation and protein aggregation link, potentially paving the way for more effective treatments.
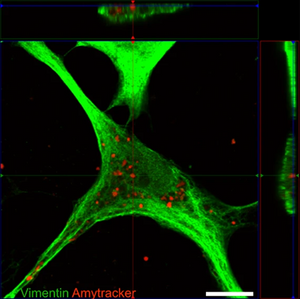
Image: Confocal micrograph showing an astrocyte with internalised tau fibrils. Vimentin (a component of the cytoskeleton) was stained using an green-fluorescent antibody, while the tau aggregates are stained with Amytracker 680 (red). Image from Figure 2 B by Eltom et al. (2024) Acta Neuropathologica Communications, 12, 34 (CC BY 4.0)
Read More:
- Chandhok et al. (2023) Scientific Reports, 13:14471
- Eltom et al. (2024) Acta Neuropathologica Communications, 12, 34
- Li et al. (2024) eLife, 13, RP92350.
- Hofbauer et al. (2023) Immunity, 54(8), 1772-1789.
- UN Department of Economic and Social Affairs, World Social Report 2023.
- Stephenson et al. (2018) Immunology, 154(2), 204-219
- Mullard et al. (2019) Nature Reviews Drug Discovery, 18, 327
- Zhou et al. (2020) PLOS One, 15(3)
- Orti-Casan et al. (2022) PNAS, 119(37).
