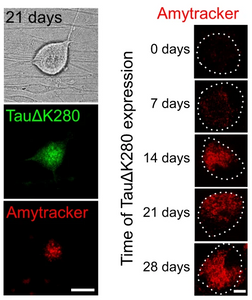
Accumulation of abnormal tau protein in the brain has been described as pathological hallmark of a group of neurodegenerative disorders called tauopathies. The most common tauopathy is Alzheimer’s disease (AD). In AD, intracellular inclusions containing aggregates of hyperphosphorylated tau coexist with extracellular amyloid plaques containing aggregated amyloid-β (Aβ). Clinically, tau inclusions correlate with cognitive impairment and therefore tau pathology is considered to be the major driver for neuronal degeneration and has become a target of rapidly evolving therapeutic strategies. Tau plays an important physiological function in dynamically interacting with axonal microtubules and is the target of various post-translational modifications. It is crucial that therapeutic agents conserve, or even restore the physiological function of tau, and prevent propagation of a range of abnormal forms of tau including soluble oligomers. Cell-based models of tauopathies are in high demand, as a platform for monitoring tau aggregation testing and efficacy of therapeutic compounds. A study published in the journal Nature Communications by Pinzi et al., describes a live-cell imaging assay to identify compounds that restore physiological microtubule interaction of an aggregation-prone full-length human tau construct in axon-like processes of model neurons and axons of primary neurons. The researchers used viral a single amino acid deletion mutant TauΔK280 of the tau gene which showed greater than 50% increased aggregation compared to wild-type tau in a heparin-induced cell-free aggregation assay to create a construct with an N-terminal photoactivatable GFP (PAGFP) reporter sequence. They successfully transfected neuronal cells (PC12) and primary neurons (DRG) with a viral vector and showed reduced interaction with microtubules of mutant TauΔK280 in the cellular environment using a technique called fluorescence decay after photoactivation (FDAP), likely due to oligomer formation. While initially, no amyloid forms of tau were identified in the cells using Amytracker 680, cells that were imaged 3 weeks after transfection showed accumulation of amyloid tau and Amytracker staining of cell bodies over time indicate significant formation of tau amyloids after 14 days of transfection.
After establishing their cellular model, the researchers proceeded to test the efficacy of potential therapeutic agents preventing tau aggregation. A compound called PHOX15 was able to restore mutant TauΔK280 interaction with microtubules, likely by preventing oligomer formation. While PHOX15 was not able to reverse aggregation, once amyloids were present, it was able to reduce the formation of tau amyloids in neurons. Taken together, the researchers showed that PHOX15 reduces the formation of tau filaments and decreases the phosphorylation of tau at disease-relevant sites thereby restoring the interaction of tau with microtubules to a physiological level. Their cell based model for testing and careful characterization of the compound distinguish PHOX15 as a promising therapeutic target and may advance therapeutic approaches that specifically target the key traits of tauopathies.
Image: Presence of tau amyloids in DRG neurons. Left: PAGFP-TauΔK280 and Amytracker 680 fluorescence indicate formation of tau amyloids in the neuronal cell body after 21 days (scale bar: 20 µm). Right: Amytracker 680 signal indicates the formation of tau amyloids in the neuronal cell body on day 0-28 (scale bar: 20 µm). Image from Figure 2DE by Pinzi, L. et al. (2024) Nature Communications, 15(1), 1679 (CC BY 4.0).
