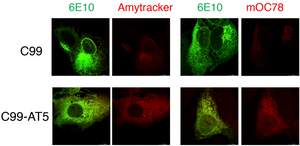
What causes the accumulation of toxic amyloid aggregates? An article by the group of Bingwei Lu from the Department of Pathology at Stanford University School of Medicine published in the journal Acta Neuropathologica Communications might have found the answer to this question. Their work shows that a defective ribosome quality control (RQC) causes the C-terminal fragment (APP.C99) of the Amyloid precursor protein (APP) to accumulate. This might be one of the earliest pathogenic events of amyloid aggregation. Labelling such aggregates in HeLa cells with Amytracker 680 shows that they have amyloid-like properties.
Image: Ribosome stall-induced APP.C99 with C-terminal extensions seeds amyloid ß-42 aggregation in HeLa cells. Fluorescent images showing aggregation of Aß42 (labeled by 6E10 antibody, green) as detected by Amytracker (left, red) as well as mOC78 antibody (right, red).Image from Figure S6F by Rimal, S. et al. (2021) Acta Neuropathologica Communications, 9, 169 (CC BY 4.0)
