Parkinson's disease (PD) and Multiple System Atrophy (MSA) are both characterized by accumulation and misfolding of ɑ-synuclein (ɑ-syn). However, there are distinct differences between their pathology likely relating back to different strains of ɑ-syn aggregates. The goal of a study by De Luca et al. from IRCCS and SISSA in Italy was to analyze how neuronal SH-SY5Y cells reacted to different forms of ɑ-syn aggregates isolated from the olfactory mucosa of patients with PD and MSA as well as healthy controls using a Seed Amplification Assay. While ɑ-syn isolates from PD and MSA patients showed an efficient seeding activity, those from healthy controls did not. Seeded aggregates were labeled with an MJFR anti-alpha-synuclein aggregate antibody and showed that there is a certain structural difference between aggregates seeded with ɑ-syn isolated from PD and MSA olfactory mucosa, also confirmed by different grades of resistances to proteinase K. Analyzing a panel of inflammatory molecules, the researchers found that the inflammatory response was based on TLR2 activation and that the inflammatory response caused by MSA seeded aggregates was more pronounced. To better understand and analyze the structural differences between different ɑ-syn aggregate strains, the researchers generated ɑ-syn aggregate strains by fibrillating recombinant ɑ-syn under different conditions (ɑSv1, ɑSv2, ɑSv3). Structural differences in recombinant ɑ-syn strains were confirmed by Proteinase K treatment, MJFR antibody staining as well as a dye binding assay using Thioflavin T, Bis-ANS, CongoRed and all Amytrackter variants.
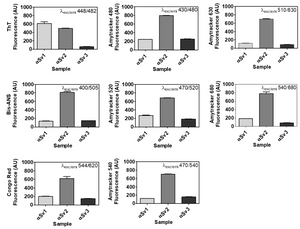
Image: Dye-binding assay of recombinant α-Syn aggregate strains. Once generated, αSv1, αSv2 and αSv3 were incubated with ThT, Bis-ANS, Congo red, Amytracker 480, Amytracker 520, Amytracker 540, Amytracker 630, Amytracker 680. αSv1 interacted with higher efficiency with ThT than αSv2 and αSv3. αSv2 interacted with higher efficiency with all the other fluorophores while αSv3 weakly interacted with all the fluorescent probes. Image from Figure S1 by De Luca et al. (2022) Cells, 11(1), 87 (CC BY 4.0).
Although capable to efficiently seed aggregation, the recombinant ɑ-syn strains did not transmit their seed-specific properties to the reaction products, which showed comparable biochemical properties. However, when used to stimulate SH-SY5Y cells, αSv1, αSv2, and αSv3 acted on different activators of inflammatory pathways, thus strengthening the existence of a correlation between morphological and inflammatory properties of αSyn fibrils.
