Faulty α-synuclein (α-Syn) aggregates in cells forming toxic α-Syn oligomers and finally Lewy Bodies which are the pathological hallmark of synucleinopathies. While Lewy Bodies are relatively easy to discern in a microscope, it turns out to be very difficult to investigate the early-intermediate forms of aggregates.
A study published in the journal Nature Neuroscience presents an assay to gain insight on the early events of α-Syn aggregation. In their paper Choi et al. make use of a phenomenon called Förster resonance energy transfer (FRET) which can assess to detect if light sensitive molecules are in very close contact to each other; because only then, the resonance energy can be transferred from a resonance energy donor to an acceptor. With modern microscopy techniques, scientists can excite a pair of fluorophores and detect FRET at a single-molecule level (smFRET), effectively turning FRET into a “spectroscopic ruler” to discern information about the conformational dynamics of various biomolecules, or the intramolecular distances amongst their building blocks.
Choi et al. make use of the smFRET technology by labelling mutated α-Syn with fluorescent tags acting as resonance energy donor/acceptor pair so that fluorescence emitted by the resonance acceptor, can be used as a proxy for α-Syn aggregation. They show that several α-Syn mutants show a concentration and time-dependent increase in the FRET signal and are able to track the structural changes of α-Syn from monomer, loose oligomers to dense higher FRET-efficiency oligomers. While monomers-oligomers appeared within hours, high-FRET oligomers only appeared after several days of incubation.
Correlating their fluorescence images with electron-micrographs by using Correlative Light Electron Microscopy, the researchers were able to show that the seeding events for α-Syn aggregation preferentially occur at the mitochondrial membrane. They further confirmed this result by showing that the signal from Amytracker co-localized with the signal of a fluorescent probe targeting cardiolipin present in the mitochondrial membrane.
In a neuronal cell line, they used Amytracker to confirm that α-Syn interacts with cardiolipin. As shown in their in vitro experiments, this interaction facilitates α-Syn aggregation, and traps cardiolipin in the growing aggregates. The accumulation of α-Syn aggregates on the mitochondria and the loss of cardiolipin can lead to an impairment of mitochondrial bioenergetics and induce mitochondrial dysfunction, which has been identified to be a major source of toxicity and the primary cause of neurodegeneration.
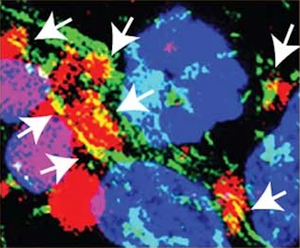
Image: In confocal images from neuronal cells, α-Synyclein aggregates are labelled with Amytracker 540 (red) and are shown to be in close contact with cardiolipin in the mitochondrial membrane (green). Image from Extended Data Figure 7 by Choi, M.L., Chappard, A., Singh, B.P. et al. (2022) Nature Neuroscience, 25, 1134-1148 (CC-BY-4.0).
Read More:
- Choi, M.L., Chappard, A., Singh, B.P. et al. (2022) Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity. Nature Neuroscience, 25, 1134–1148
- Sasmal, D.K. et al. (2016) Single-molecule fluorescence resonance energy transfer in molecular biology. Nanoscale 48, 19928-19944
