Prion diseases are fatal neurodegenerative disorders caused by the misfolding and aggregation of prion proteins (PrP). Although copper (Cu2+) imbalance has long been suspected to play a role in these conditions, a recent study by Juliani and colleagues at the Federal University of Rio de Janeiro sheds new light on this issue. Their researchreveals that Cu2+ promotes the formation of liquid-like PrP condensates that naturally buffer excess copper and help prevent toxicity. However, under oxidative stress, these condensates transition from a liquid to solid state, forming amyloid-like aggregates that may contribute to disease progression. These findings suggest a critical link between Cu2+ dysregulation, oxidative stress, and prion pathology.
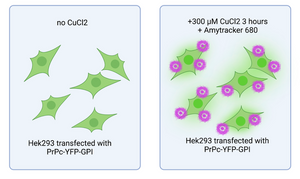
Detecting and characterizing prion aggregates is key to understanding their impact on neurodegeneration. In this study, the researchers used Amytracker 680 to visualize amyloid-like PrP aggregates in Cu2+-treated cells, confirming that prolonged exposure to Cu2+ leads to PrP aggregation - a process that mirrors the pathological changes seen in prion diseases.This work not only highlights the potential of Amytracker as a tool for tracking protein aggregation, but also supports further research into neurodegenerative disorders and potential therapeutic strategies.
Image: Figure illustrating live-cell imaging of HEK293 cells expressing PrPC-YFP-GPI. Cells treated with 300 μM CuCl2 for 3 hours (right panel) show AmyTracker680-positive aggregates on the cell surface. Image created using BioRender.
