Protein aggregation is traditionally seen as a pathological process, hallmark of neurodegenerative diseases and systemic amyloidoses. However, emerging evidence reveals that amyloid aggregation is not merely the consequence of protein misfolding; rather, cells harness reversible amyloid aggregation to adapt to environmental stress. Understanding how functional amyloids form and disassemble could provide critical insights into the treatment of pathological aggregation.
The team led by Timothy Audas at Simon Fraser University, has recently published its work on Amyloid bodies (A-bodies) - nuclear amyloid-like inclusions that sequester proteins in response to environmental stressors. Similar to other stress-related inclusion (e.g. cytoplasmic stress granules) the composition of A-bodies is stress-specific. This suggests that cells dynamically regulate the recruitment of proteins into A-bodies based on environmental conditions. Notably, several disease-associated proteins, such as β-amyloid peptides, are recruited and aggregate within A-bodies, indicating a potential link between pathological amyloidogenesis and dysregulation of A-body formation.
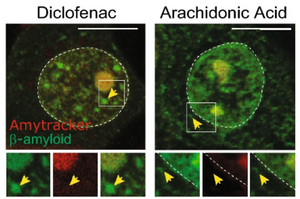
The study highlights how small molecules that reduce the aggregation of β-amyloid in vitro had no effect on the recruitment of pathological fragments A-bodies. On the other hand, diclofenac, a nonsteroidal anti-inflammatory drug, despite having no effect in traditional in vitro assays, significantly reduced the amount of β-amyloid aggregates formed in response to stress. Importantly, the drug does not impair the broader formation of A-bodies, underscoring the specificity of its effects.
These findings position A-bodies as a model for studying amyloid aggregation in a cellular context. Tools like Amytracker, which enable real-time monitoring of live-cell aggregation, are critical for advancing this field. By bridging the gap between in vitro studies and physiological conditions, the A-body model opens pathways for identifying novel therapeutic compounds.
Image: Amytracker 680 (in red) was used to stain β-Amyloid (1-42)-GFP expressing MCF-7 cells treated with either Diclofenac or Arachidonic Acid and exposed to acidosis treatment. In both cases, Amytracker stained the Amyloid body in the nucleus, but not the cytoplasmic inclusions of β-Amyloid. Image adapted from Figure 4 by Chandhock, S., Pereira, L., Momchilova, E.A. et al. (2023) Scientifc Reports, 13, 14471. (CC BY 4.0).
