Studying the inflammation-aggregation connection using Amytracker
By 2050, the number of people aged >65 is expected to double. This will lead to a significant rise in the prevalence of age-related diseases. Many of these conditions are linked to two major processes: protein aggregation and chronic inflammation.
The precise relationship between these two components and how they contribute to the pathology of age-related diseases remains unclear. It is uncertain whether protein aggregation triggers inflammation, or if inflammation drives the aggregation process.
To date, anti-amyloid therapies, which were designed to reduce protein aggregation, have not delivered the hoped-for results in clinical trials. Instead, targeting the pro-inflammatory signalling pathways has shown promise at the pre-clinical level. It seems that this strategy could hold the key to developing effective therapies against neurodegenerative diseases.
One major challenge for researchers is the lack of tools to observe and visualise early stages of protein aggregation within live cells and organisms. A recent publication by Eltom et al. found that astrocytes can take up amyloid fibrils from their surroundings, but are unable to degrade them. Instead, the fibrils accumulate in the astrocytes' cytoplasm, causing the release of pro-inflammatory cytokines. Similar findings were reported by the Klenerman group at Cambridge University and the Bruns group at the University Hospital in Erlangen. They showed that macrophages activate pro-inflammatory signaling when exposed to either amyloid-β fibrils or β2-microglobulin (which accumulates in multiple myeloma). >Research from the Audas lab at Simon Fraser University demonstrated that anti-inflammatory drugs can reduce amyloid-β accumulation, further strengthening the relationship between inflammation and protein aggregation.
These findings underscore the value of Amytracker as a tool designed for live-cell studies which could provide crucial insights into the relationship between inflammation and protein aggregation link, potentially paving the way for more effective treatments.
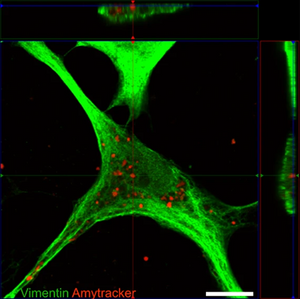
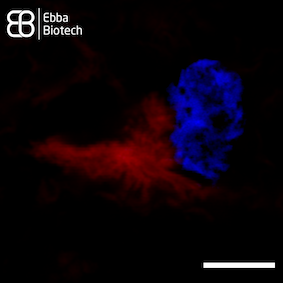
Image: Confocal micrograph showing an astrocyte with internalised tau fibrils. Vimentin (a component of the cytoskeleton) was stained using an green-fluorescent antibody, while the tau aggregates are stained with Amytracker 680 (red). Image from Figure 2 B by Eltom et al. (2024) Acta Neuropathologica Communications, 12, 34 (CC BY 4.0)
Read More:
Multi-Laser / Multi-Detector Imaging with Amytracker
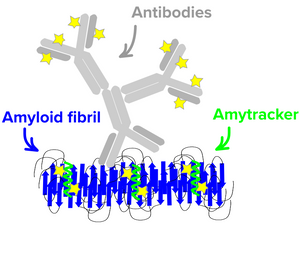
Amytracker are optotracers with structure-dependent photo-physical properties. All Amytracker variants are designed to bind to the Congo red binding pocket on the amyloid fibril and require a theoretical minimum of eight in-register parallel-β-strands for binding. Therefore, Amytracker reliably labels amyloids derived from a variety of amyloidogenic proteins or peptides from different species. Due to their structure-dependent photo physical properties, the Amytracker variants are only fluorescent when binding to a target and different targets can produce a difference in the molecules fluorescence spectrum. To investigate different targets, we recommend to perform imaging by exciting the sample with different wavelengths collecting fluorescence intensity in multiple emission ranges (see the table below for reference). Excitation- and emission spectra for all Amytracker variants can be accessed here.
| Channel | Excitation | Emission range | Amytracker variant |
|---|---|---|---|
| CH1 | 405 nm | 400-490 nm | Amytracker 480 Amytracker 680 |
| CH2 | 405 nm | 490-600 nm | Amytracker 480 Amytracker 680 |
| CH3 | 405 nm | 600-660 nm | Amytracker 480 Amytracker 680 |
| CH4 | 405 nm | 660-700 nm | Amytracker 480 Amytracker 680 |
| CH5 | 488 nm | 500-580 nm | Amytracker 520 Amytracker 540 Amytracker 630 |
| CH6 | 488 nm | 580-650 nm | Amytracker 520 Amytracker 540 Amytracker 630 |
| CH7 | 488 nm | 650-700 nm | Amytracker 520 Amytracker 540 Amytracker 630 |
| CH8 | 561 nm | 600-650 nm | Amytracker 630 Amytracker 680 |
| CH9 | 561 nm | 650-700 nm | Amytracker 630 Amytracker 680 |
| CH10 | Brightfield | ||
Optotracing using Amytracker
Amytracker are optotracers with structure-dependent photo-physical properties. All Amytracker variants are designed to bind to the Congo red binding pocket on the amyloid fibril and require a theoretical minimum of eight in-register parallel-β-strands for binding. Therefore, Amytracker reliably labels amyloids derived from a variety of amyloidogenic proteins or peptides from different species. Due to their structure-dependent photo-physical properties, the Amytracker variants are only fluorescent when binding to a target and different targets can produce a difference in the molecules fluorescence spectrum. To investigate different targets, we recommend collecting excitation and emission spectra with excitation and emission parameters summarised in the table below. Reference spectra for all Amytracker variants can be accessed here.
| Exmax | Emmax | Excitation spectrum (detect at Emmax) | Emission spectrum (excite at Exmax) | Recommended filter-sets |
|
|---|---|---|---|---|---|
| Amytracker 480 | 420 nm | 480 nm | 300 - 450 nm | 450 - 800 nm | DAPI |
| Amytracker 520 | 460 nm | 520 nm | 300 - 490 nm | 490 - 800 nm | FITC, GFP |
| Amytracker 540 | 480 nm | 540 nm | 300 - 510 nm | 510 - 800 nm | FITC, GFP, YFP |
| Amytracker 630 | 520 nm | 630 nm | 300 - 600 nm | 550 - 800 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
| Amytracker 680 | 530 nm | 680 nm | 300 - 650 nm | 660 - 800 nm | PI, mCherry, Cy3.5 |
Amytracker and Long Covid
Around 30% of the people infected with the SARS-CoV-2 virus report persistent symptoms for a long time after the acute infection ends. While the development of this long-term manifestation after COVID, referred to as Long COVID, has been framed as mysterious, it is actually a well described outcome of many viral or bacterial infections. In the case of Long COVID, the chronic symptoms include shortness of breath, fatigue, chest pain, headaches, “brain fog”, and a procoagulant state.
However common, it is not clear how the virus mediates these prolonged effects. Several hypotheses have been proposed to try and explain the phenomenon: Some experts propose that SARS-CoV-2 is able to reach and infect the cells of the central nervous system and infect neurons, thus causing neuroinflammation. Others believe that the virus might remain active in certain tissues and not be cleared completely from the body. Finally, it has been suggested that the virus affects the microbiome and the endothelium, allowing bacteria to enter the bloodstream.
This last hypothesis is particularly interesting in light of the work of Prof. Resia Pretorius from the University of Stellenbosch in South Africa. In her early career, she worked on the “bacteria in blood hypothesis” suggesting that bacteria from the gut and the oral mucosa might exist in the blood in a dormant state. During intense periods of stress, these bacteria might exit dormancy and shed inflammagens, such as LPS or other Endotoxins, causing generalized inflammation and abnormal clotting involving the formation of fibrin amyloids, which can be identified using Amytracker. As abnormal clotting has been linked to several inflammatory diseases like Diabetes and Parkinson's Disease by Pretorius et al (Read more here), it provides a connection between inflammation and protein aggregation, which can be identified using Amytracker. When the COVID-19 pandemic hit, Prof. Pretorius turned to the blood of COVID patients, and found that the infection caused the formation of the same kind of abnormal blood clots that she described before. These amyloid clots can reach 200 μm in size, meaning that they can effectively block microcapillaries and thus reduce the oxygen supply to tissues. A reduction in oxygen availability could, by itself, explain many of the symptoms of both acute and long COVID, providing a way in which a predominantly respiratory disease could lead to damage to organs like the kidney, the muscles, and the central nervous system.
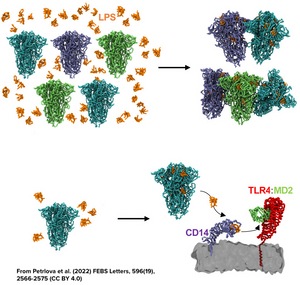
In addition, the work from Nyström & Hammarström from Linköping University in Sweden has shown how certain regions of the spike protein produced by SARS-CoV-2 have amyloidogenic properties, while Petrlova et al. from Prof. Schmittchen’s group at the University of Lund in Sweden recently demonstrated that LPS can trigger the aggregation of the spike protein as a whole in structures that are labeled using Amytracker (See feature image and read more here)
Taken together, these findings indicate that Long COVID might be an amyloid disease or amyloid-related disease caused by hyperinflammation as well as the presence of viral components and bacterial inflammagens in the blood. Although these findings are not conclusive and a diagnostic assay for Long COVID is still to be developed, we might consider abnormal clotting and amyloid formation as one of the puzzle pieces towards solving the Long COVID mystery.
Read More:
Amytracker and the age-pigment Lipofuscin
The macromolecules and organelles within cells are not permanent and need to get replaced over time. To do this, cells need to both produce new components and to degrade the old ones. The degradation of the bigger cellular structures depends on a process called autophagy. During autophagy, the cell envelopes the to-be-destroyed organelle and uses lysosomes to digest it down to its basic components (amino acids, sugars, lipids, etc.). Unfortunately, lysosomes cannot completely degrade all the substances the organelles are made of, and this defect results over time in the formation of lipofuscin. In cells that are actively dividing, lipofuscin is diluted through the generations, but in long-lived cells that do not divide anymore - like neurons and cardiomyocytes - it continuously accumulates in cytoplasmic granules, and ultimately determines the apoptotic death of the cell. For these reasons, lipofuscin is generally known as the “age-pigment” and its accumulation is the most prominent cytological manifestation of the ageing process.
Lipofuscin shows a broad spectrum of autofluorescence that varies among different tissues. Within the brain, lipofuscin shines mostly yellow light when excited at 330 to 370 nm. It exhibits a broad, gently-sloping, emission spectrum that covers wavelengths between 480 and 660 nm, with an emission maximum between 540 and 570 nm.
When imaging tissue with heavy Lipofuscin fluorescence together with Amytracker 520 or Amytracker 540, we recommend to use a confocal laser scanning microscopy with availability of various options for excitation and emission. When emission is collected in four channels (blue: 400-480nm, green: 480-600 nm, yellow: 600-650 nm, red: 656-700 nm), it is possible to excite at 405 nm or 488 nm. Excitation at 405 nm has the advantage that lipofuscin will localise close to the nuclei, when a DAPI counterstain is used. Amytracker fluorescence would not be very bright due to excitation far from the excitation maximum. When excitation is performed at 488 nm, nuclei won’t be visible, but Amytracker fluorescence will be much brighter, which would allow to omit Lipofuscin fluorescence completely by modulating brightness or laser power. (Image: Fresh Frozen tissue section from temporal lobe tissue with AD pathology, fixed in-ice cold EtOH and labelled with Amytracker 520 and DAPI. Left: 405 nm excitation Right: 488 nm excitation, scale: 50 µm)
Read More:
Amytracker for the study of tau aggregates
Tau is an intracellular protein which associates with microtubules and stabilizes them. Physiologically, Tau is phosphorylated to facilitate release from the microtubules and thereby favor microtubule shortening. In a pathological state, Tau is hyperphosphorylated which increases its tendency to aggregate in the cytoplasm. This means that conditions which promote abnormal phosphorylation of Tau promote its aggregation. Aggregates of hyperphosphorylated Tau form insoluble filaments and tangled clumps. These intracellular deposits are called Neurofibrillary tangles (NFTs).
In the literature, predecessors of Amytracker have been used to label NFTs in tissue sections. It was found that the signal co-localises with the signal from monoclonal antibodies for phosphorylated Tau (AT8), thus confirming its specificity.
In cortical tissue sections showing pronounced AD pathology Amytrackers labels bona-fide intracellular aggregates and reveals a clear fibrillar morphology assembled in tangled net-like structures (see image).
Read More:
Amytracker for studying α-synuclein aggregates
Aggregates of α-synuclein are the major component of Lewy bodies which are the pathological hallmark of a series of neurodegenerative disorders, called Lewy body diseases or Synucleinopathies. In physiological conditions, α-synuclein regulates synaptic vesicle-release and possibly cytoskeletal assembly. However, this small pre-synaptic protein is characterized by an intrinsically disordered structure that makes it prone to aggregation. When aggregated, α-synuclein can interact with membranes and, for instance, make neurotransmitter vesicles “leaky”. This is especially important in dopaminergic neurons, as the interaction between dopamine and α-synuclein seems to further facilitate its oligomerization. When aggregated, α-synuclein cannot be disposed of by the cellular recycling pathway, it is instead deposited into intracellular inclusion bodies together with other proteins.
An Amytracker-like molecule has been used to label α-synuclein aggregates amplified in cerebrospinal fluid from patients with Synucleinopathies such as Multiple Systems Atrophy and Parkinson’s Disease (Shahnawaz, M. et al, 2020). Further, Amytracker 680 has been used to confirm the amyloid nature of α-synuclein aggregates in a cellular fibril-seeding assay (Mahul-Mellier, A. et al. 2020).
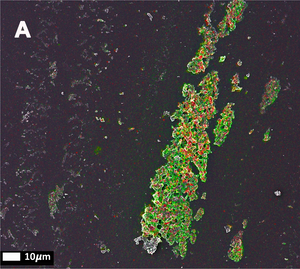
Superresolution images of thalamic tissue sections with pronounced PD pathology labeled with Amytracker 680 and DAPI counterstain show intracellular aggregates (red) located close to the nucleus (blue) in great detail (see image, scale bar 5 µm).
When proteins get out of shape
Human cells typically assemble a myriad of different proteins which constitute the work-force of the cellular environment and each of them is dedicated to a very specific function. The ability of a protein to perform its task is closely related to its structure: pockets, arms, and fingers are needed to store, move, and grasp molecules and other proteins resembling the workings of tiny biological machines.
All proteins are translated from messenger RNA into a sequence of amino acids. In order to perform their specific task, they have to fold and assemble into a specific three-dimensional structure. The instructions for proper folding are encoded in the amino acid sequence. Each amino acid carries different charged groups favouring certain interactions. Thus, a protein that contains errors in its sequence might struggle to fold properly. Since the charge of the amino acid is also related to its chemical microenvironment, folding might be compromised under oxidative or acidic conditions. Moreover, the folding process sometimes just fails, even when there are no sequence or environment changes.
Misfolded proteins expose portions of themselves that are normally buried on the inside, and thus interact with their environment in an abnormal way. Especially dangerous are exposed hydrophobic regions. They can compromise the cell compartmentalization by interacting with the membranes of organelles and vesicles; or they can stick together, forming compact fibrils that we call “amyloid”, due to their resemblance to starch (lat.: amylum). In these amyloid fibrils, the misfolded proteins interact non-specifically with each other using their amino acid backbone, rather than the residues that are normally exposed to the surface. This results in the formation of extensive β-sheets, held together by a large number of hydrogen bonds, that are very stable and almost impossible to break down. Moreover, amyloid fibrils seem to be able to trap other proteins and seed their conversion into more amyloid structures i.e. spreading. Even though it entails loss of function, aggregation is thought to have a protective effect: the more misfolded proteins interact with each other and aggregate, the less they are available for interaction with membranes or available for spreading.
Protein misfolding manifests itself in a plethora of diseases. They are collectively called amyloidoses, since they are associated with the deposition of amyloids in form of extracellular plaques or intracellular inclusions which may present systemically, or localised. The type and localisation of aggregates can be traced back to the protein that is affected by misfolding. It is still unclear, however, if the aggregates themself play a role in the pathology, or if they are symptomatic of an underlying issue.
The most well-studied amyloid diseases are the neurodegenerative diseases Alzheimer’s disease (AD) and Parkinson’s disease (PD). In AD, deposition of amyloids in form of extracellular plaques as well as intracellular neurofibrillary tangles is observed. The extracellular deposits can be led back to abnormal proteolytic cleavage of the Amyloid Precursor Protein (APP) which produces amyloidogenic Aβ peptides which form extracellular deposits in close proximity to the synaptic cleft. Intracellular deposits observed in patients with AD originate from a hyperphosphorylated form of tau protein. Soluble tau protein normally contributes in regulating microtubule assembly. Under pathological conditions, it becomes hyperphosphorylated and prone to aggregation forming insoluble filaments and tangled clumps, known as neurofibrillary tangles (NFTs). NFTs accumulate inside the neuronal cell body and survive even after the death of affected neuronal cells, where they may be released extracellularly. In PD, amyloid aggregates are mostly intracellular and appear as spherical masses in the cytoplasm. These have first been described by Fritz Heinrich Lewy in 1910 and are since called Lewy bodies. Their major component is α-synuclein. In physiological conditions, α-synuclein regulates synaptic vesicle-release and possibly cytoskeletal assembly. However, this small pre-synaptic protein is characterised by an intrinsically disordered structure that makes it prone to aggregation. When aggregated, α-synuclein can interact with membranes and, for instance, make neurotransmitter vesicles “leaky”. This is especially important in dopaminergic neurons, as the interaction between dopamine and α-synuclein seems to facilitate further α-synuclein oligomerization. This might be the reason why dopaminergic neurons of the substantia nigra in the midbrain are the first ones to degenerate in the patients. This particular region of the brain has a role in suppressing involuntary movements, and its loss is responsible for the characteristic tremors.
Many more forms of amyloid diseases exist and to date, 37 human proteins have been found to form amyloid deposits in pathology. While it is under debate, if the amyloid aggregates cause or contribute to the pathology, they are an important hallmark for the diagnosis of underlying disease. Due to their involvement in debilitating and progressive diseases, amyloids are associated with a negative connotation in general. However, a plethora of functional amyloids exists in humans and other organisms.
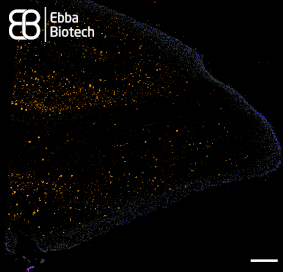
With our family of Amytracker molecules, Ebba Biotech provides a toolbox for sensitive, fast and safe detection of functional and disease-associated amyloid aggregates (See image showing a temporal lobe tissue section with pronounced AD pathology labelled with Amtracker 680 and DAPI counterstain. Tiled image at 10X magnification (Ex: 405 nm, Em1: 415-456 nm, Ex2: 587-694 nm), scale bar: 1mm).
Read More:
Are amyloid structures in abnormal blood clots a risk factor for amyloidosis?
Amyloidosis is the name for a group of conditions caused by a build-up of amyloid protein deposits in organs and tissues throughout the body. A great many diseases can be classified as amyloidosis. The most well known are the cerebral amyloidoses Alzheimer's and Parkinson's disease. Lesser known are the systemic amyloidoses and Type-2-Diabetes. Although some genetic determinants have been identified, the large majority of patients suffering from amyloidosis have no identified family history, meaning the disease is sporadic and acquired. As symptoms in the early stages of the disease are often diffuse and mimic those of other conditions, diagnosis is difficult. Therefore, identification of risk factors is an important tool that can be used to facilitate early diagnosis. While it is well known that patients with a chronic infectious or inflammatory disease may be at greater risk of developing secondary amyloidosis, the link between inflammation and protein aggregation hasn’t been well described.
A series of publications from the research group around Prof. Erethesia Pretorius at Stellenbosch University in South Africa describes the relationship between inflammation and abnormal coagulation. Their studies propose that abnormal coagulation might be the missing link between inflammation and protein aggregation. Prof. Pretorius’ early work has been based on the finding that many supposedly non-communicable diseases like stomach ulcers actually have a bacterial or viral origin. A bacterial link has also been proposed in the aetiology of Alzheimer’s disease, Parkinson’s disease and even implicated in Type-2-Diabetes. Earlier work of Prof. Pretorius together with Prof. Douglas B. Kell at University of Manchester in the United Kingdom describes how dormant bacteria, unrecognized by microbiological testing, can circulate in the blood and exit from dormancy through a dysregulation of iron metabolism and/or stress. The reactivation from dormancy can release bacterial inflammagens that lead to an activation of the immune system and cause an inflammatory response, which is linked to abnormal clotting or hypercoagulation caused by amyloidogenic fibrin(ogen).
In the first paper out of a series of three (de Waal, G.M. et al. (2018)), the authors identified a well known bacterial inflammagen - lipopolysaccharide (LPS) - associated with fibrin fibres in blood clots of patients suffering from Parkinson’s disease, Alzheimer’s disease and Type-2-Diabetes. The authors obtained whole blood samples from patients and healthy controls and prepared platelet poor plasma (PPP) which they used to induce formation of PPP clots by adding thrombin. They used Amytracker 480, Amytracker 680 and Thioflavin T to detect amyloids in PPP clots from healthy individuals or individuals with Parkinson’s disease and found that there is more amyloid when clots are formed with fibrin(ogen) of diseased individuals than with fibrin(ogen) of healthy individuals. On top of that, they were able to detect elevated amounts of the bacterial inflammagen LPS in PPP or whole blood smears from diseased patients. Using an advanced imaging technique called correlative light electron microscopy, which allows the overlay of a confocal or super-resolution (fluorescence) micrograph onto a scanning electron micrograph, the authors showed that the fluorescent signal obtained from an anti-LPS antibody is merged and fused into the dense matter of the fibrin(ogen) deposits.
The second publication in the series (Adams, B. et al. (2019)) builds on these findings and investigates the link between Parkinson’s disease and a bacterial inflammagen - gingipain - a protease produced by Porphyomonas gingivalis (P. gingivalis), which is common in the oral cavity with the capacity to cause chronic periodontitis. Gingipains, like other bacterial inflammagens, are present on the bacterial surface, but can also be secreted from the bacterium and can enter the circulation. The results of the study showed that whole blood from Parkinson’s disease patients contains elevated levels of pro-inflammatory biomarkers and cytokines. This goes along with platelets showing substantial (hyper)activation, spreading and aggregation together with a tendency for hypercoagulation in whole blood samples. When green fluorescent fibrinogen was incubated with P. gingivalis LPS and treated with thrombin to form clots, fibrin networks display a denser and more matted network. The protease gingipain alone greatly inhibited fibrin network formation, but the effect was limited when incubated together with P. gingivalis LPS. A fluorescent antibody was used to detect gingipain in blood samples and it was evident that gingipain was present in clots obtained from PPP of diseased patients. As also shown in the previous publication, clots obtained from PPP of diseased patients show enhanced fluorescence when labeled with Amytracker 480, Amytracker 680 or Thioflavin T. This result suggests that in clots derived from the blood of Parkinson’s disease patients, fibrinogen polymerizes into a form with greatly increased number of β sheets, resulting in the formation of amyloid fibrils.
The third publication in the series (Page, M.J. et al. (2019)) takes a step away from bacterial inflammagnes and investigates the role of inflammation on anomalous blood clotting. As a marker for inflammation, the authors use the acute-phase protein Serum Amyloid A (SAA). In response to inflammatory processes, cytokines induce SAA production in the liver resulting in up to 1000-fold increase in SAA plasma concentration. At the site of infection, SAA activates the inflammation cascade leading to activation of the innate immune response. In the study, the authors added purified SAA to green fluorescent labeled fibrinogen as well as whole blood or platelet poor plasma (PPP) from healthy donors. The results confirm the observations made using bacterial inflammagens. SAA promoted atypical coagulation and platelet activation. When SAA was added to fibrinogen and clotted with thrombin, amyloid structures were identified after labeling with Amytracker 680, Amytracker 480 or Thioflavin T. When SAA was added to green fluorescent labeled fibrinogen and clotted with thrombin, large areas of hypercoagulable fibrin(ogen) were found. Amytracker 680 was shown to bind in the vicinity of these hypercoagulable areas, pointing towards the fact that anomalous hypercoagulated areas that form in the presence of SSA have an amyloid nature. When SAA was added to PPP of healthy donors, and clots were obtained by adding thrombin, fluorescence of the amyloid markers Amytracker 680, Amytracker 480 and Thioflavin T increased and showed large patches of visible amyloid. Correlative light electron microscopy was used to show close association of SAA, labeled using a green fluorescent antibody, and amyloid structures, labeled by Amytracker 680 (Figure from Page, M. et al. (2019) Correlative Light Electron Microscopy confirms the presence of SSA in amyloidogenic fibrinogen clots, CC BY 4.0).
Taken together, the publications present evidence that bacterial inflammagens or innate inflammatory proteins in the circulation lead to anomalous clotting and the formation of amyloid structures in fibrin(ogen) clots. These amyloid structures seem to be associated with the causative inflammagens. Amyloid structures in plasma clots from patients with cerebral or systemic amyloidosis closely resemble those caused by various inflammagens and specific bacterial inflammagens can be detected in anomalous clots from diseased patients. These results strongly point towards the fact amyloid aggregates in abnormal blood clots might present a risk factor for amyloidosis.
Read More:
Phase separation and protein aggregation
Phase separation of biomolecules has recently been recognised as an important cellular process governing homogeneous organisation which is a driving force for cellular self-assembly. Multivalent interactions between biomolecules give rise to condensed phases with a spectrum of material properties from liquids to solids. The liquid-like properties (wetting, fusion, and dynamic exchange of internal components) of membraneless organelles such as P granules, stress granules, and the nucleolus have been demonstrated to be based on Liquid–liquid phase separation. The process of protein aggregation - disordered proteins which pathologically self-assemble into insoluble fibres that further aggregate into the plaques, tangles, or inclusions has long been believed to be related to a common, systemic origin and mechanism of toxicity. The potential for disordered proteins to assemble via phase separation into soft condensed material states raises questions about the relationship between phase separation and neurodegeneration. The evidence in support of a relationship between droplet properties, protein aggregation, and neurodegenerative pathology is rapidly growing.
Protein liquid–liquid phase separation (LLPS) is studied in cellular subcompartments like membraneless organelles or in vitro. Protocols for protein LLPS in vitro depend on the studied proteins since different proteins undergo LLPS under different conditions according to their properties. Generally, LLPS can be achieved by decreasing the ionic strength of the buffer, addition of precipitators, co-addition of interacting partners, and removal of the recombinant tag by protease. Several assays and standards are performed to determine whether the proteins undergo LLPS, including assessing the turbidity of the solution, microscopic examination of liquid droplets, monitoring the fusion and fission of liquid droplets, assessing their wetting properties, and subjecting the mixture to sedimentation to analyze the partitioning of proteins between the different phases.
Researchers from Max Planck Institute of Biochemistry in Martinsried, Germany have recently published an article (Frottin, F. et al. (2019)) in the renowned scientific journal Science where they study protein aggregation in the nucleolus, which is the largest non–membrane bound subcompartment in the nucleus. The nucleolus consists of liquid-like phases that do not intermix, giving rise to distinct zones. They found that, during stress, misfolded proteins enter the liquid-like GC phase of the nucleolus, where irreversible co-aggregation of different misfolded protein species is prevented, allowing Hsp70-mediated extraction and refolding (or degradation) upon recovery from stress. In contrast, disruption of the GC phase causes the formation of stable protein aggregates which they detected using Amytracker 680. Prolonged stress resulted in a transition of the nucleolar matrix from liquid-like to solid and prevents nucleolar quality control.
Read More:
Amytracker fluorescence spectra
We named our Amytracker molecules after their peak emission wavelength when they are bound to their target. That means, when Amytracker is bound to a target, it will emit fluorescence at peak emission indicated by the number associated with its name.
To view the excitation and emission spectra, please select your Amytracker below :
Amytracker compared to Congo Red
Amytracker fluorescence is one order of magnitude brighter than Congo Red. The affinity of Amytracker is in the nM range and thus, Amytracker is typically used at several fold lower concentrations. In a comparative study using human amyloidosis tissue, Amytracker detected amyloid deposits in 15 % of Congo Red negative samples. While the pathological relevance of Congo Red negative deposits is currently unclear, Amytracker staining might give an indication of earlier states of disease. Unlike Congo Red, Amytracker does not bind to collagen or other cytoskeletal proteins. In the APP/PS1 and APP23 mouse models of Alzheimer's disease, Amytracker stains early fibrils already after 6 months, while Congo Red stains mainly mature fibrils showing up after 12 months in the APP/PS1 mouse model. Amytracker has also been utilized to detect disease-associated protein aggregates, such as prion proteins and inclusion bodies, which go undetected by Congo red.
Read More:
Does Amytracker bind unspecifically?
We tested a wide range of human tissues and didn't observe unspecific staining in most cell types. Positive Amytracker staining was obtained in Paneth cell granules in the intestine stained and the binding target in these cells is yet unclear. Due to the high sensitivity of Amytracker towards amyloids, and its ability to detect Congo Red negative and non-thioflavinic assemblies, Amytracker might serve as an interesting tool to study functional amyloids in different species.
Read More:
Fixation technique for Amytracker
Generally, we recommend light fixation using ice-cold ethanol or acetone. This is because formalin-fixation has been shown to reduce the ability to stain inclusion bodies. Otherwise there shouldn't be any issues. You can use paraffin sections or cryosections. Note that epitope exposure and antigen retrieval is not needed when applying Amytracker to paraffin embedded sections.
Read More:
Amytracker for use in various tissues and species
It is likely that Amytracker works on all kinds of tissues and species. Amytracker targets and detects the physical topography of the tertiary- and quaternary structure of mature and pre-fibrillar amyloid deposits. As such it is applicable to a wide range of animal models and different Amytracker molecules have been applied on tissue sections from humans, mice, cows, sheep, deer and Drosophila.
Amytracker binding
All Amytracker molecules are designed to interact with the same binding site on the amyloid oligomer. Competition assays have shown that Amytracker competes for the congo red binding site but show much higher affinity. In essence the binding cavity and binding mode of Amytracker is is dictated by a groove lined with repetitive positive charged side-chains. The detection of prefibrillar species appears dependent on 8 repetitive β sheets, composed of a minimum of eight in-register parallel-β-strands.
Read More:
Amytracker for detection of Amyloidosis
Amytracker have been shown to bind with high affinity to aggregates composed of transthyretin (TTR) in Drosophila models of transthyretin amyloidosis (ATTR), as well as in human tissues The optotracers have been used to detect aggregates in following forms of human amyloid diseases: AA amyloidosis associated with accumulation of serum amyloid A (SAA) protein, isolated atrial amyloidosis associated with accumulation of atrial natriuretic factor, various forms of amyloid light chain (AL) amyloidosis and islet amyloid polypeptide (IAPP) or amylin which is believed to be of critical importance for the loss of β-cells in type 2 diabetes.
Read More:
Amytracker compared to Thioflavin
When Amytracker are not bound to a target, they exhibit an extremely low background fluorescence. Amytracker have also been shown to neither accelerate nor inhibit amyloid formation when used in recommended (substochoimetric) concentrations. Therefore, Amytracker are suitable for fibrillation assays and spectrophotometric detection. Amytracker have been shown to identify pre-fibrillar non-thioflavinophilic assemblies during in vitro fibrillation of Aβ peptides, insulin, lysozyme and prion protein with significantly reduced lag-phase compared to Thioflavin.
Read More:
How should I dilute Amytracker?
We supply Amytracker molecules with high affinity toward amyloid proteins. For staining of cryo- or paraffin sections, diluting the supplied solutions 1:1000 should be sufficient. If you want to increase the intensity, you might increase the concentration and use 1:500 dilution instead. For live cell staining, we usually recommend to dilute our products 1:500. For fibrillation assays and spectroscopic application you should titrate the supplied optical tracer molecules, so you work with substochiometric amounts and make sure that there is not too much unbound probe in your sample.
Amyloids - the dark matter of biology
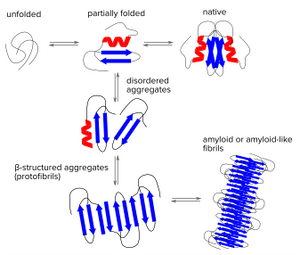
Under some conditions, peptides or proteins may convert from their soluble forms into unsoluble highly ordered fibrillar aggregates. These aggregates may cause disease through various mechanisms. Prominent examples of aggregating peptides related to neurodegenerative diseases are Amyloid β peptides which play an important role in Alzheimer's disease and aggregating α-synuclein which is found in Lewy bodies that are a hallmark of Parkinson's disease and some forms of dementia. Multiple variants of systemic amyloidoses are caused by aggregating immunoglobin light chains, fragments of serum amyloid A protein or aggregating transthyretin. But many more forms of systemic and localized amyloid diseases exist.
Protein folding is a delicate process in which partially folded polypeptide structures are vulnerable to forming disordered aggregates. These disordered structures might dissociate again, but under certain conditions they will get stuck and form very stable β structured aggregates (protofibrils) which can grow into mature fibrils by further self-association or repetitive addition of monomers. Thus, regardless of the aggregating polypeptide chains share any sequence homology, the β-sheet is the structural hallmark of many types of fibrillar aggregates called Amyloids. Heterogenic features of amyloids might be due to the alignment of adjacent strands and the separation of the amyloid structures.
Read More:
Amytracker for amyloid staining
Amyloid detection has been notoriously difficult since current methods are either laborious, toxic and/or tend to detect mature fibrils but not protofibrils or premature aggregates.
Amytracker are fluorescent tracer molecules binding to amyloids with high sensitivity. Amytracker have been shown to bind to prefibrillar states of amyloids and might therefore help to detect and investigate earlier pre-pathological states of amyloid diseases. As Amytracker are fluorescent only when bound to their target, they are well suited for fibrillation studies with much shorter lag phase and less background compared to other small molecule ligands. Amytracker are non-toxic and are well suited for life-cell applications crossing cell membranes with minimal incubation times.
Amytracker to investigate amyloid formation
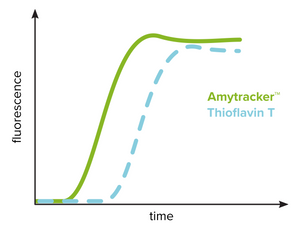
The kinetics of amyloid formation from conformational conversion of a peptide or protein into its fibrillar form (amyloid) is studied using fibrillation assays using a spectrophotometer. This technique requires extremely low background fluorescence of the unbound probe and Thioflavin T has been widely used for this reason. The kinetic profile typically includes a lag phase that is followed by a rapid exponential growth phase and a plateau phase. As shown in the figure, Thioflavin T can reliably identify the presence of amyloid fibrils but are limited in detecting prefibrillar aggregates. Amytracker binds and detects prefibrillar aggregates present during the initial lag phase as well as mature amyloid fibrils. Therefore, the lag phase is shortened significantly when investigating amyloid formation using Amytracker. Amytracker has been used to study fibrillation of recombinant Aβ1-40 and Aβ1-42 as well as lysozyme and insulin.
Read More:
Amytracker for super-resolution microscopy
Super-resolution microscopy is becoming an important tool to study biological structures. As super-resolution techniques like STED overcome the physical diffraction limit of light, new microscopes with ever-decreasing resolution limits are being developed. Using these exciting techniques, the constraints are now imposed by the probes used for labelling. With STED microscopy, reaching a resolution of 20–40 nm, antibodies are no longer suitable as labelling probes since conjugated fluorophores will seem to be located far away from their target and spatial constraints will lead to spotty images.
Due to their small size of less than 1 kDa and high affinity our fluorescent tracer mlecules are excellently suited for binding-activated localization microscopy (BALM). Since they are only fluorescent when binding, they can be localized with high precision before turning dark due to photobleaching. Amytracker have been used to obtain <20 nm resolution images of unlabeled α-synuclein fibrils using BALM.
Read More:
Amytracker for live cell Imaging
As functional aspects of amyloids as well as the dynamic processes involving amyloid formation and amyloid toxicity are of growing interest many researchers are interested to study these processes in living cells. Non-invasive techniques like fluorescence microsopy have been perfected in recent years for the study of living cells. Unfortunately, few non-toxic fluorescent dyes with high affinity to amyloids exist.
Amytracker have been shown to readily pass membranes of living cells such as dorsal root ganglion cells and human pancreatic islet cells and bind intracellular protein aggregates. No cytotoxic effects have been observed even with high concentrations of Amytracker. For use in living cells, we recommend to dilute Amytracker 1:500 in cell culture medium and incubate cells for 30 min under cell culture conditions. We developed the deep red Amytracker 680 specifically for live cell applications to avoid interference with autofluorescence common in living cells and tissues. Amytracker 540 is our green/yellow fluorescent tracer molecule specifically designed for superior uptake into cells and tissues.
Read More:
In vivo amyloid staining and intravital imaging
Intravital imaging is allowing researchers to capture images of biological processes in live animals. It has become an advanced tool to study the progression of Alzheimer's and other neurodegenerative diseases in transgenic mice. In vivo imaging using two-photon microscopy is an advantageous technique for observing tissues and organs at high resolution. Amytracker are suitable for in vivo studies since our probes are non-toxic, able to cross the blood-brain barrier and stain fibrillar deposits in animal models of amyloidogenic diseases.
Presently, we supply Amytracker - Solid in sterile injection bottles optimized for labeling of protein aggregates in vivo for intravital imaging of protein aggregates. We recommend diluting Amytracker - Solid in physiological saline or PBS to 4 mg/ml and injecting a total dose of 5mg/KG intravenously. Imaging is optimal 24-72h